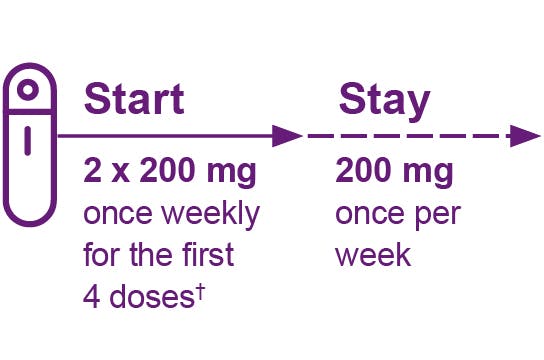
NARRATOR:
Hi there. Thank you for joining me as I show you, step by step, how to use the pre-filled autoinjector for BENLYSTA, also known as belimumab, for once weekly use under the skin only, also known as subcutaneous use. Please remember that you should receive training from a healthcare professional before injecting BENLYSTA for the first time. At the end of this video, we will review the important safety information for BENLYSTA.
ON-SCREEN TEXT:
Instructions for Use Video
BENLYSTA (belimumab) Subcutaneous Use 200 mg/mL
Please review the Important Safety Information
for BENLYSTA after these instructions.
NARRATOR:
To make things easier, I will put important notes on the side of your screen so you can follow along. In the package insert of your BENLYSTA autoinjector, you will find the medication guide as well as the Instructions for Use. Read the full instructions on how to inject BENLYSTA, and refer to them throughout the injection process. Be sure to follow the instructions on how to use the autoinjector the right way. If you don’t, the autoinjector may not work properly. The autoinjector is for use under the skin only, also known as subcutaneous use.
ON-SCREEN TEXT:
Instructions for Use Video
Look at your package insert for full instructions on how to inject BENLYSTA.
ON-SCREEN TEXT:
Important Storage Information
NARRATOR:
Please take note of the following important storage information for the BENLYSTA autoinjector. Keep it refrigerated until 30 minutes before use. Keep it in the original package until time of use to protect it from light.
ON-SCREEN TEXT:
Important Storage Information
- Keep refrigerated until 30 minutes before use.
- Keep in the original package until time of use to protect it from light.
NARRATOR:
Do not freeze BENLYSTA. Do not expose BENLYSTA to heat and sunlight. Do not use BENLYSTA and do not place it back in the refrigerator if left at room temperature for more than 12 hours. Keep BENLYSTA out of the reach of children.
ON-SCREEN TEXT:
Important Storage Information:
- Do not freeze.
- Do not expose to heat and sunlight.
- Do not use and do not place back in the refrigerator if left at room temperature for more than 12 hours.
- Keep out of the reach of children.
NARRATOR:
Please take note of the following important warnings.
ON-SCREEN TEXT:
Important Warnings
NARRATOR:
The BENLYSTA autoinjector should be used only once and then disposed of safely in a sharps container.
ON-SCREEN TEXT:
Important Warnings:
- The BENLYSTA autoinjector should be used only once and disposed of safely in a Sharps Container.
NARRATOR:
Do not share your autoinjector with another person. You may give other people a serious infection or get a serious infection from them. Do not shake the autoinjector. Do not use it if dropped onto a hard surface. Do not remove the Ring Cap until just before the injection.
ON-SCREEN TEXT:
Important Warnings
- Do not share your autoinjector with another person. You may give other people a serious infection or get a serious infection from them.
- Do not shake.
- Do not use it if dropped onto a hard surface.
- Do not remove the Ring Cap until just before the injection.
ON-SCREEN TEXT:
Supplies Needed for the Injection
NARRATOR:
Now it’s important to know all of the autoinjector parts so you know what to look for when following along. The autoinjector contains an Inspection Window and a Grey Stopper, which you will need to watch during the injection. Later when it is time to remove the Ring Cap, you will see a Gold Needle Guard, but the needle is not visible. The autoinjector also has an expiration date. It’s important that you do not use the autoinjector if the expiration date has passed.
ON-SCREEN TEXT:
Supplies Needed for the Injection
Autoinjector Parts:
- Inspection Window
- Grey Stopper
- Ring Cap
- Gold Needle Guard
- Expiration Date
NARRATOR:
There are some supplies you will need to perform your injection. In addition to your BENLYSTA autoinjector, you’ll need an alcohol swab, a gauze pad or cotton ball, and a sharps container for autoinjector disposal. These materials are not included in the BENLYSTA package, so make sure you have them on hand before you inject.
ON-SCREEN TEXT:
Supplies Needed for the Injection
BENLYSTA autoinjector
- Alcohol Swabs (not included)
- Gauze Pad or Cotton Ball (not included)
- Sharps Container (not included)
NARRATOR:
If you call BENLYSTA Cares at 1-877-4-BENLYSTA (1-877-423-6597), they’ll send you a free sharps container, carrying case, and BENLYSTA User’s Guide. They also offer ongoing injection support by phone.
ON-SCREEN TEXT:
Call BENLYSTA Cares at 1-877-4-BENLYSTA
(1-877-423-6597) to get a free Sharps Container, carrying case, and BENLYSTA User’s Guide.
NARRATOR:
Now that you have your supplies, grab a fresh autoinjector from the fridge and set yourself up in a comfortable space – whatever’s easiest and most comfortable for you. Where you set up should also be well-lit, with a clean surface where you can place the autoinjector, alcohol swab, gauze pad or cotton ball, and the sharps container within easy reach. If you don’t have these items, do not self-inject. But if you do, let’s get started.
ON-SCREEN TEXT:
Supplies Needed for the Injection:
- Find a comfortable space to do the injection.
- Make sure you have a clean, well-lit surface.
- Place the autoinjector, alcohol swab, gauze pad or cotton ball, and sharps container within reach.
- Do not perform the injection if you do not have all the supplies shown here.
ON-SCREEN TEXT:
Injection Preparation
NARRATOR:
Simply peel back the film of the tray and carefully remove the autoinjector. Now it’s time to prepare and inspect your autoinjector.
ON-SCREEN TEXT:
Injection Preparation
Peel back the film of the tray and remove the autoinjector.
NARRATOR:
Check if the expiration date has passed. If it has, do not use it. Warm up the autoinjector by letting it sit at room temperature for 30 minutes – be sure not to warm it up any other way.
ON-SCREEN TEXT:
Injection Preparation
- Check the expiration date on the autoinjector.
- Do not use if the expiration date has passed.
- Allow the autoinjector to sit at room temperature for 30 minutes.
- Do not warm up the autoinjector in any other way.
NARRATOR:
Do not put it in the microwave or hot water or in direct sunlight. And do not remove the Ring Cap during this step.
ON-SCREEN TEXT:
Injection Preparation
- Do not warm in a microwave oven.
- Do not warm in hot water.
- Do not warm in direct sunlight.
- Do not remove the Ring Cap during this step.
NARRATOR:
If you are doing the injection with me, it’s time to let your autoinjector sit at room temperature for 30 minutes. Once 30 minutes have passed, come back and we will continue with the injection.
ON-SCREEN TEXT:
Injection Preparation
Allow the autoinjector to sit at room temperature for 30 minutes.
30 min
NARRATOR:
Okay, pause the video and I’ll see you in 30 minutes.
ON-SCREEN TEXT:
Pause for 30 Minutes
NARRATOR:
After 30 minutes, look at the Inspection Window to check that the autoinjector solution is colorless to slightly yellow in color. It is normal to see one or more air bubbles in the solution. Do not use if the solution looks cloudy, discolored, or has particles.
ON-SCREEN TEXT:
Injection Preparation
After 30 minutes, look in the Inspection Window to check that the autoinjector solution is colorless to slightly yellow in color.
It is normal to see one or more air bubbles in the solution.
Do not use if the solution looks cloudy, discolored, or has particles.
NARRATOR:
Now it’s time to make an important decision. Where on your body will you make your injection? There are only a couple of places we are allowed to inject: in the abdomen or in the thighs. Keep in mind that you want to avoid injecting into the same exact spot each time. And try to avoid areas where the skin might be tender, bruised, red, or hard.
ON-SCREEN TEXT:
Injection Preparation
Choose an injection site (abdomen or thigh).
Avoid injecting into the same site each time, and avoid areas where the skin is tender, bruised, red, or hard.
NARRATOR:
And do not inject within 2 inches of the belly button.
ON-SCREEN TEXT:
Injection Preparation
Do not inject within 2 inches of the belly button.
NARRATOR:
You may have a hard time remembering where to inject yourself next. If you call BENLYSTA Cares at 1-877-4-BENLYSTA (1-877-423-6597), or sign up on benlystatools.com, they will send you free injection stickers in the mail within six to eight weeks. You can mark each tray of BENLYSTA with these stickers. You can also buy color-coordinated stickers of your own or mark the trays with a permanent marker.
ON-SCREEN TEXT:
Injection Preparation
Use injection stickers to help you remember where to inject yourself next.
Call 1-877-4-BENLYSTA (1-877-423-6597) or visit benlystatools.com to request your stickers and other useful tools.
NARRATOR:
Once you’ve decided on an injection site, it’s time to wash your hands. When your hands are clean, wipe the injection site with an alcohol swab. Wait a little bit, and allow the skin to air dry.
ON-SCREEN TEXT:
Injection Preparation
Wash your hands.
NARRATOR:
Do not touch this area again before giving the injection.
ON-SCREEN TEXT:
Injection Preparation
- With clean hands, clean the injection site by wiping it with an alcohol swab.
- Allow the skin to air dry.
Do not touch this area again before giving the injection.
NARRATOR:
Now it’s time to prepare for the actual injection. When you’re ready, remove the Ring Cap. Never remove the Ring Cap before this point.
ON-SCREEN TEXT:
Injection Preparation
Do not remove the Ring Cap until right before you give the injection.
NARRATOR:
You can remove the Ring Cap by pulling or twisting it off. The Ring Cap may be twisted off in either a clockwise or counterclockwise direction. Once you’ve removed it, do not put the Ring Cap back onto the autoinjector.
ON-SCREEN TEXT:
Injection Preparation
- Remove the Ring Cap by pulling or twisting it off.
- The Ring Cap may be twisted off in either a clockwise or counterclockwise direction.
ON-SCREEN TEXT:
Injection Process
NARRATOR:
The positioning of the autoinjector is another important thing to focus on. Hold the autoinjector comfortably so you can view the Inspection Window and see the purple indicator moving while giving the injection. This is important so that you can confirm a complete dose.
ON-SCREEN TEXT:
Injection Process
Hold the autoinjector comfortably so you can view the Inspection Window and see the purple indicator moving while giving the injection.
NARRATOR:
If you need to, create a flat area for the injection site by pulling or stretching the skin like this.
ON-SCREEN TEXT:
Injection Process
If needed, create a flat area for the injection site by pulling or stretching the skin.
NARRATOR:
Position the autoinjector straight over the injection site, and make sure the Gold Needle Guard is flat on the skin. You want to create a 90-degree angle with the autoinjector and your skin.
ON-SCREEN TEXT:
Injection Process
Position the autoinjector straight over the injection site at a 90-degree angle. Make sure the Gold Needle Guard is flat on the skin.
NARRATOR:
Now you are ready to inject. Firmly press the autoinjector all the way down onto the injection site and hold it in place.
ON-SCREEN TEXT:
Injection Process
Firmly press the autoinjector all the way down onto the injection site and hold it in place.
NARRATOR:
This will insert the needle and start the injection. You may hear a “first click” at the start of the injection, and you will see the purple indicator start to move through the Inspection Window. Continue to hold the autoinjector until the purple indicator has stopped moving.
ON-SCREEN TEXT:
Injection Process
- This will insert the needle and start the injection.
- You may hear a “first click” at the start of the injection, and you will see the purple indicator start to move through the Inspection Window.
- Continue to hold the autoinjector until the purple indicator has stopped moving.
NARRATOR:
The injection may take up to 15 seconds. You may hear a “second click” a few seconds before the purple indicator stops moving. Just keep holding the autoinjector down until the purple indicator has completely stopped.
ON-SCREEN TEXT:
Injection Process
You may hear a “second click,” but continue to hold the autoinjector down until you see that the purple indicator has completely stopped.
15 sec
NARRATOR:
When the injection is complete, lift the autoinjector from the injection site.
ON-SCREEN TEXT:
Injection Process
When the injection is complete, lift the autoinjector from the injection site.
ON-SCREEN TEXT:
After the Injection
NARRATOR:
Once the injection is done, do not put the Ring Cap back onto the autoinjector.
ON-SCREEN TEXT:
After the Injection
- Do not put the Ring Cap back onto the autoinjector.
NARRATOR:
Now you can place your used autoinjector and Ring Cap into a sharps container right away after use. Do not throw them away in your household trash. If you do not have an FDA-cleared sharps container, please review the “BENLYSTA Instructions for Use” document for information on household containers that can be used as a sharps container.
ON-SCREEN TEXT:
After the Injection
- Put your used autoinjector and Ring Cap in a Sharps Container.
- If you do not have an FDA-cleared Sharps Container, you can refer to the BENLYSTA Instructions for Use for information on household containers that can be used as a Sharps Container.
NARRATOR:
You can now inspect your injection site. You may see a small amount of blood. If needed, simply press a cotton ball or gauze pad on the injection site. And remember, do not rub the injection site.
ON-SCREEN TEXT:
After the Injection
There may be a small amount of blood at the injection site.
Do not rub the injection site.
NARRATOR:
Always keep your Sharps Container out of the reach of children.
ON-SCREEN TEXT:
After the Injection
Always keep your Sharps Container out of the reach of children.
NARRATOR:
When your Sharps Container is almost full, ask your healthcare provider how to dispose of it properly. There may be state or local laws you need to follow about throwing away used needles and syringes. Don’t throw away your used Sharps Container in your household trash, unless your community guidelines allow you to, and don’t recycle your used Sharps Container. For more information about safe sharps disposal, including sharps disposal laws in the state where you live, go to fda.gov/safesharpsdisposal.
ON-SCREEN TEXT:
After the Injection
When your Sharps Container is full:
- Ask your healthcare provider how to dispose of your Sharps Container.
- Follow all state and local laws.
- Do not throw away your used Sharps Container in your household trash.
- Do not recycle your used Sharps Container.
- For more information about safe sharps disposal in your state, go to: http://www.fda.gov/safesharpsdisposal.
NARRATOR:
Now you know how to properly inject BENLYSTA. But before you go, let’s review the Important Safety Information for BENLYSTA.
NARRATOR:
What is BENLYSTA?
BENLYSTA is a prescription medicine used to treat people with active systemic lupus erythematosus (SLE or lupus) or active lupus nephritis (lupus-related kidney inflammation) who are receiving other lupus medicines. It’s unknown if BENLYSTA is safe and effective in children under 5 years when given in a vein or under 18 years when given under the skin. BENLYSTA is not for people with severe active central nervous system lupus.
ON-SCREEN TEXT:
What is BENLYSTA?
BENLYSTA is a prescription medicine used to treat people with active systemic lupus erythematosus (SLE or lupus) or active lupus nephritis (lupus-related kidney inflammation) who are receiving other lupus medicines. It’s unknown if BENLYSTA is safe/effective in children under 5 years when given in a vein or under 18 years when given under the skin. BENLYSTA is not for people with severe active central nervous system lupus.
NARRATOR and ON-SCREEN TEXT:
Important Safety Information
Do not use BENLYSTA if you are allergic to belimumab or any ingredients in BENLYSTA.
The most important information about BENLYSTA
Immunosuppressive agents, including BENLYSTA, can cause serious side effects. Some of these may cause death.
- Infections: fever, chills, pain or burning with urination, urinating often, coughing up mucus, or warm, red, or painful skin or sores on your body. Infections could be serious, leading to hospitalization or death.
- Allergic (hypersensitivity) reactions: itching, swelling of the face, lips, mouth, tongue, or throat, trouble breathing, anxiousness, low blood pressure, dizziness or fainting, headache, nausea, or skin rash. Serious allergic reactions can happen on the day of, or in the days after, receiving BENLYSTA and may cause death.
- Mental health problems and suicide: thoughts of suicide or dying, attempt to commit suicide, trouble sleeping (insomnia), new or worse anxiety or depression, acting on dangerous impulses, other unusual changes in your behavior or mood, or thoughts of hurting yourself or others.
Before receiving BENLYSTA, discuss with your healthcare provider if you:
- think you have an infection or have infections that keep coming back. Do not use BENLYSTA if you have an infection unless your healthcare provider tells you to.
- have or have had mental health problems such as depression or thoughts of suicide.
- have recently received or may need a vaccination. If you are receiving BENLYSTA, you should not receive live vaccines.
- are taking any medicines, including prescription, over-the-counter, vitamins, and herbal supplements.
- are allergic to other medicines.
- are receiving other biologic medicines.
- have or have had any type of cancer.
- have any other medical conditions.
- are pregnant or plan to become pregnant. It is unknown if BENLYSTA will harm your unborn baby. Talk to your healthcare provider about whether to prevent pregnancy while on BENLYSTA. If you choose to prevent pregnancy, you should use an effective method of birth control for at least 4 months after the final dose of BENLYSTA.
- become pregnant or if you think you may be pregnant while on BENLYSTA. Talk to your healthcare provider about enrolling in the BENLYSTA Pregnancy Registry to monitor the health of you and your baby. You can enroll in this registry by calling 1-877-681-6296.
- are breastfeeding or plan to breastfeed. It is unknown if BENLYSTA passes into your breast milk.
Possible side effects of BENLYSTA
- Progressive multifocal leukoencephalopathy (PML). PML is a serious and life-threatening brain infection. PML can result in death or severe disability. Tell your healthcare provider right away if you notice any new or worsening medical problems: memory loss, trouble thinking, dizziness or loss of balance, difficulty talking or walking, or loss of vision.
- Cancer. Medicines that affect the immune system, including BENLYSTA, may increase your risk of certain cancers.
The most common side effects of BENLYSTA are nausea, diarrhea, fever, stuffy or runny nose and sore throat, persistent cough, trouble sleeping, leg or arm pain, depression, headache, and pain, redness, itching, or swelling at the site of injection (when given subcutaneously). These are not all the possible side effects of BENLYSTA. Call your doctor for medical advice about side effects.
NARRATOR:
If there was anything you didn’t understand about using the autoinjector, or the injection process, just remember that this video will always be here for your reference. If you have any additional questions, please ask your doctor or nurse.
ON-SCREEN TEXT:
Additional Information
- You can ask your healthcare provider or pharmacist for information about the BENLYSTA autoinjector that is written for healthcare professionals.
NARRATOR:
Remember, you can also contact BENLYSTA Cares at 1-877-4-BENLYSTA (1-877-423-6597). Or visit benlystatools.com to request a free BENLYSTA User’s Guide. You can also get ongoing injection support by phone. Take care and bye for now.
ON-SCREEN TEXT:
Additional Information
- Call BENLYSTA Cares at 1-877-4-BENLYSTA (1-877-423-6597) or visit benlystatools.com for a free BENLYSTA User’s Guide and ongoing injection support.
- You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or 1-800-FDA-1088
ON-SCREEN TEXT:
[GSK logo] ©2023 GSK or licensor. BELVID230020 October 2023 Produced in USA.